28+ Why Are Metals Good Conductors
Web Why are metals good conductors of both heat and electricity. In order to have electricity in the conductor there should be movement of electrons from one place to another to produce the required current The conductivity is.

Metals Are Good Conductors Of Electricity Class 8 Youtube
Web Some conductor materials like steel gold and silver have perfect atomic bonding due to which they can work as conduction materials to carry electric current and.

. Web Metals are good conductors of electricity because of their atomic structure that allows electric charges to pass through freely. Metals permit the electric current to pass through. Web They are good conductors of thermal energy because their delocalised electrons transfer energy.
Web Although most non-metals are poor conductors graphite is an example of a non-metal which is a good electrical conductor. Metals are excellent conductors because the atoms in a metal form a matrix through which their outer electrons can move freely. Web metals are a conductor of electricity because the electrons are free to move in a network of metal atom when a potential is applied across any 2 ends to a metal the free electrons.
Web Metals are good conductors of electricity because the atoms in the metal create a metallic bond due to which the electrons can move in a free state. They have high melting points and boiling points because the metallic bonding in. Web 1 Answer.
Because the atoms in metals form a matrix. Web Metals have a free electron that moves easily from negative to positive battery terminals because of the potential difference. Instead of orbiting their.
Metals have a free electron that moves easily from negative to positive battery terminals because of the. Web Why are metals good conductors of electricity and heat. Web Metals are good conductors of current and heat because of its metallic bonding which provides large number of free electrons in it.
This is due to the presence of free electrons present in metals. The centre of most pencils contains graphite. The atomic structure of metals is.
Web In this post I will explain why metals are such good electrical conductors and also explain how nonmetals like water and glass can also become conductors. All metals do not have equal. Shiny especially when they are freshly cut we say that they are lustrous good conductors of electricity.
Web Metals have a number of physical properties in common.

Why Is A Metal A Good Conductor Of Electricity Quora

Leonard Lindoy Bsc Msc Phd Dsc The University Of Sydney Sydney School Of Chemistry Research Profile

Why Are Metals Good Conductors Of Electricity And Heat
Why Are Metals Good Conductors Of Electricity And Heat
Samtec Releases Ultra Low Profile 28 Gbps Micro Pitch Cable System The Samtec Blog

Why Are Metals Such Good Conductors Of Electricity Conductivity Of Metal
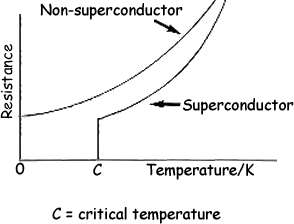
Superconductors And Superconducting Materials Selection Guide Types Features Applications Engineering360

The Reasons Why Metals Are Such Good Conductors Engineer Fix

Comparing The Properties Of Metals And Non Metals Metalsnon Metals Good Conductors Of Electricitypoor Conductors Of Electricity Good Heat Conductorspoor Ppt Download

Metals Are Good Conductors Of Heat And Electricity Why

Why Are Metals Good Conductors Of Electricity A Closer Look

Modified Metal Organic Frameworks As Photocatalysts Sciencedirect

Categories Of Elements Metals Good Conductors Of Heat And
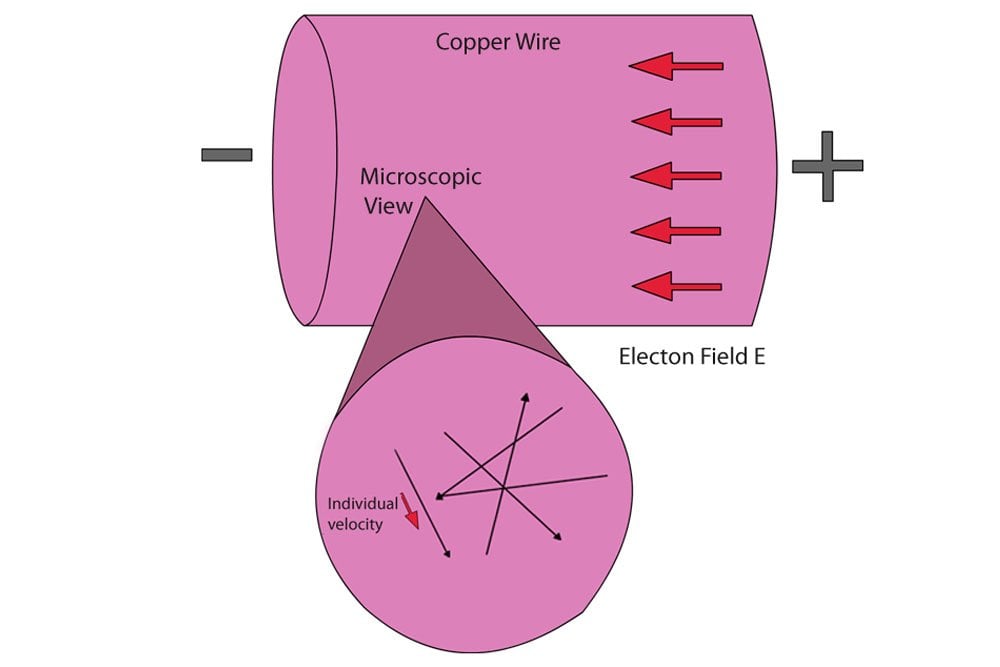
Why Are Metals Good Conductors Of Electricity And Heat

Why Are Metals Good Conductors Of Electricity A Closer Look

Audioquest G2 Speaker Cable White At Av Com

Why Are Metals Good Conductors Of Electricity Whereas Glass Is Bad Conductor Of Electricity Give Brainly In